Tired of reading about politics? Don’t have anything else to do on a Friday afternoon? Why not brush up on metric units and unit conversions?
No, really! Click below for more.
American units vs. the metric system
In America, we’re used to using so-called “English” or “US customary” units. This is unfortunate because these units are irregular – and thus harder to use. Scientists and most of the rest of the developed world use the metric system (the “SI” system). The main advantage of metric units is that they’re constructed in such a way that most units can be derived from simple base units. The SI base units we see most often are shown below.
| Quantity | SI base unit | Symbol |
|---|---|---|
| Length | meter | m |
| Mass | kilogram | kg |
| Temperature | Kelvin | K |
| Time | second | s |
These base units are not the only units you’ll use in metric, but many other units can be defined in terms of these base units.
(The kilogram is a bit of an oddity for those new to metric. It’s the base unit of mass in the SI system, but it has a prefix. We usually treat the gram as if it is the base when we do unit conversions.)
Temperature: Celsius, Fahrenheit, and Kelvin
The three temperature scales you’ve probably encountered are the Fahrenheit scale, the Celsius scale, and the Kelvin scale. In the US, we use the Fahrenheit scale most of the time. In science, we use the Celsius and Kelvin scales regularly and rarely use the Fahrenheit scale.
| oF | oC | K | Notes |
|---|---|---|---|
| -459.67 | -273.15 | 0 | Absolute zero – as cold as it gets! |
| -320.44 | -195.8 | 77.35 | Boiling point of liquid nitrogen |
| -108.4 | -78.00 | 195.1 | Sublimation point of dry ice |
| -40 | -40 | 233 | An interesting temperature … |
| 0 | -18 | 255 | Zero Fahrenheit |
| 32 | 0 | 273 | Freezing point of water |
| 77 | 25 | 298 | Room temperature |
| 86 | 30 | 303 | A warm day |
| 98.6 | 37.0 | 310.2 | “Normal” body temperature |
| 212 | 100 | 373 | Boiling point of water |
| 621.43 | 327.46 | 600.61 | Melting point of lead metal |
| 1474 | 801.1 | 1074 | Melting point of sodium chloride |
These reference points should give you something of a feel for how the Celsius and Kelvin scales compare to the Fahrenheit scale. Here’s how to convert back and forth between the units.
Fahrenheit to Celsius:
![]()
Celsius to Fahrenheit:
![]()
Celsius to Kelvin:
K = C + 273.15
To get from Celsius to Kelvin you simply add a number. This means that a Celsius degree is the same size as a Kelvin degree. So why the two scales? The Kelvin scale is an absolute scale, while Celsius and Fahrenheit are not. What’s an absolute temperature scale? One that doesn’t have any negative values. Zero on the Kelvin scale is, essentially, as cold as it can get.
The metric prefixes
SI units can be scaled to meet your needs. If you’re trying to describe the length of your finger, the meter is probably too big. (Your finger might not be but 0.08 meters long!). Instead, you’d prefer to use a unit that would give you a nice round number – say, 8 centimeters. You can scale a base unit to whatever magnitude you like using one of the metric prefixes, like centi-. Here are some common metric prefixes.
| Prefix | Symbol | Multiplier |
|---|---|---|
| mega- | M | 106 |
| kilo- | k | 103 |
| deci- | d | 10-1 |
| centi- | c | 10-2 |
| milli- | m | 10-3 |
| micro- | 10-6 | |
| nano- | n | 10-9 |
| pico- | p | 10-12 |
These aren’t all of the metric prefixes, but they’re the ones you’re likely to run into.
So how do the prefixes work? Centi-, for example, means 10-2. So a centimeter is 10-2 (or 1/100) meters. Flipping it around, it takes 102 centimeters (100 centimeters) to make a meter. Kilo- means 103. A kilometer is 103 meters, or 1000 meters.
Unit conversions
So how do you convert between one unit and another? You might have been taught some shortcuts back in grade school for converting back and forth between centimeters and kilometers and so forth, but the method we will discuss now is general . It will work for all cases – including those where grade-school shortcuts will fail.
The method is called the factor-label method, or sometimes “dimensional analysis“. It’s a process of converting a quantity from one unit to another by using conversion factors . Conversion factors are equalities (or ratios) that relate one unit to another. For example, we said that the prefix centi- meant 10-2, and a centimeter was 10-2 meters. We also said that the prefix kilo- meant 103 , and a kilometer was 103 meters . We can express these relationships as conversion factors.
1 cm = 10-2 m
1 km = 103 m
These two expressions are conversion factors to convert from one unit to another. We can also express these conversion factors as ratios:
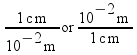
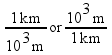
Since the numerator and denominator in these ratios are equal to each other, all of these fractions are mathematically equal to 1. Multiplying by one of these factors will change the units of a measurement, but not the measurement itself.
We can use these ratios to convert from one unit to another. For example, let’s convert a measurement of 1.205 m to cm .
![]()
The meter units cancel out and we’re left with centimeters – which is what we want. We used a conversion factor that canceled out the unwanted unit and left us with the desired one. Since multiplying by the conversion factor is mathematically equivalent to multiplying by 1, we have not changed the actual value of the measurement. All we have done is to change the unit.
Now let’s look at a trickier example. Convert a measurement of 120.5 cm to km.
You may worry that you don’t have a conversion factor that goes directly from centimeters to kilometers. You could derive one from the table of multipliers earlier in this note pack, but there’s a simpler way to deal with the problem. Convert to the base unit (the unit with no prefix) first . Then, convert from the base unit to the desired unit .
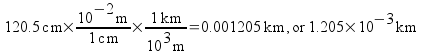
This time we cancel out centimeters to convert to meters, then we multiply by another conversion factor to convert to our desired unit, kilometers. All units cancel out except for our desired unit. The most important thing to remember when doing these conversions is to check and make sure your units cancel our properly. This means that you should write down units with every number in your calculations.
Why use this method instead of just moving decimal points around? (Metric, after all, is based on powers of ten.) Accuracy. For simple conversions involving one prefix – like converting meters to centimeters – most students can move the decimal in their head without too much trouble. But if two prefixes are involved, then less than half of students who want to move the decimal in their heads get the right answer at the end. Plus, the factor-label method can be used for more that metric – as long as you have a simple relationship between one unit and another. (In fact, this method works very well for English conversions, too!)
Derived units – volume and density
Units can be derived from base units by simply adding prefixes. Units can also be derived from base units by combining multiple units. Two of these derived units are volume and density.
The volume of an object is simply how much three-dimensional space an object occupies. In math class (a long time ago), you probably learned that the volume of a box is equal to its length times its width times its height.
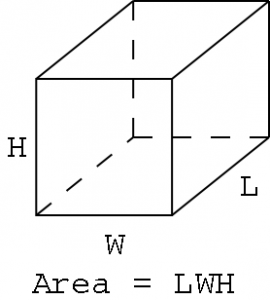 |
|
| Illustration 1 – Volume of a box |
If you measure the sides of a box with SI base units (meters), you’ll find that the area has a unit of cubic meters, or m3. The cubic meter is the SI unit of volume, and it equals the volume of a cube one meter on each side. This is a large volume – a meter is equal to a little over a yard – so we often scale down to a smaller volume unit. We use the liter (L) and milliliter (mL).
| 1 L = 1000 mL = 1 dm3
1 mL = 1 cm3 |
The other important derived unit we will discuss is density. Density is a term you’re probably familiar with. We say that small but heavy objects are dense. The density of an object is its mass per unit volume. In base SI units, density would be expressed as kg/m3 (kilograms per meter cubed – the SI unit of mass per SI unit of volume). As with volume, though, we’re used to working with smaller units in the laboratory. In most cases, you’ll deal with density as grams per milliliter, or g/mL.
Pure water has a density (at 4 oC) of 1.000 g/mL. This is a useful reference point. You can use it to relate to other substances and get a better feel for the metric system.
Summary
We’ve discussed the basics of the metric system: base units, prefixes, and two common derived units. We’ve also talked about the factor label method for unit conversions – a powerful tool useful for doing many kinds of calculations.
And now you can go back to the rest of the blog. 🙂
Tags: Education, metric system, units

Metrics, smetrics. The only measurment I care about is: how full is my food bowl? But then, I’m a cat.
Hello, I found your page when searching Google for unit conversions. I create unit converter pages, would you please be interested in placing my website’s link (code below) on your page. It’s a new website but I am serious a lot about it. With compliments and anticipation.
Convert To dot com