The humans …
![[Humans: Rick, Patty, and Cate]](http://whenchemistsattack.com/blogfiles/merrychristmas2006_humans.jpg)
… and the felines …
![[Ash in a tree]](http://whenchemistsattack.com/blogfiles/merrychristmas2006_ash.jpg)
![[Tom in a tree]](http://whenchemistsattack.com/blogfiles/merrychristmas2006_tom.jpg)
… here at the Shrimp and Grits blog would like to wish y’all a very Merry Christmas and a Happy New Year!

The humans …
![[Humans: Rick, Patty, and Cate]](http://whenchemistsattack.com/blogfiles/merrychristmas2006_humans.jpg)
… and the felines …
![[Ash in a tree]](http://whenchemistsattack.com/blogfiles/merrychristmas2006_ash.jpg)
![[Tom in a tree]](http://whenchemistsattack.com/blogfiles/merrychristmas2006_tom.jpg)
… here at the Shrimp and Grits blog would like to wish y’all a very Merry Christmas and a Happy New Year!
Since quite a few people land on this blog while searching for a recipe for shrimp and grits, here’s a repost from Patty’s family cookbook – just in time for Christmas dinner!
![[Shrimp and grits]](http://whenchemistsattack.com/blogfiles/shrimpandgrits_patty.jpg)
Shrimp and grits
Do first
Grits:
Make enough for how many people are eating. Add salt to taste (use water to cook grits, not milk).
Optional:
Add 1/2 cup of cheddar cheese to grits while cooking so it will melt
Do second
Shrimp:
In a frying pan, mix:
Cook 5-10 minutes, but don’t overcook the shrimp.
Optional:
Stir in with shrimp before serving:
Do last
Eat and enjoy!
![[Troutman's sign]](http://whenchemistsattack.com/blogfiles/troutmans_sign.jpg)
This post will be a little different than some of my previous southern BBQ features. It’s based solely on getting BBQ as a take-out rather than eating inside the restaurant. I’ll have to apologize in advance to y’all if you were wanting to see the inside of Troutman’s.
Troutman’s in Concord is a favorite BBQ spot for my wife and mother-in-law, and we managed to stop by there long enough to get a take-out order of BBQ, sauce, and slaw.
Speaking of slaw, you have a choice of two kinds at Troutman’s. You can get a more traditional white slaw, which looks like this.
![[Troutman's white slaw]](http://whenchemistsattack.com/blogfiles/troutmans_whiteslaw.jpg)
You can also get a red slaw, which looks like this.
![[Troutman's red slaw]](http://whenchemistsattack.com/blogfiles/troutmans_redslaw.jpg)
It’s the red slaw that seems to be unique to Troutman’s. The red slaw is similar to the white slaw, but with added sweet peppers and hot sauce. (What, you thought slaw was for cooling your mouth?) I’m told that the red slaw goes well on a BBQ sandwich.
| Update! For those who aren’t reading the comments, Rev. BigDumbChimp points out that the red slaw is fairly common around Winston-Salem, although the exact ingredients are variable. (Sounds a lot like “Charleston” red rice, actually…)
Thanks, Rev. BDC! |
Now we come to the BBQ itself. Troutman’s BBQ is served chopped and dry. No sauce is cooked in with the meat.
![[Troutman's BBQ on a bun]](http://whenchemistsattack.com/blogfiles/troutmans_bbq_sandwich.jpg)
Having trouble seeing the meat? Click this link and download this 1024×768 JPG image to use as your backdrop! [173K]
The meat at Troutman’s is a little bland, without much detectable smoke flavor. Sauce is provided – a pepper sauce that tastes a lot like Texas Pete with extra vinegar. I was skeptical of the sauce at first, but I have to admit that when added to the meat it makes a pretty darned good BBQ sandwich.
If you’re passing through Concord, give Troutman’s a try. Just make sure you have plenty of sweet tea handy!
Here’s a can of salted peanuts from Young Pecan.
![[Peanuts]](http://whenchemistsattack.com/blogfiles/peanutswithpeanuts.jpg)
Okay, the ingredients are … unsurprisingly … peanuts and peanut oil.
But I’d never have known that this can of peanuts, that contains peanuts and peanut oil, was made in a factory that processes peanuts without the aid of the helpful allergen statement at the bottom!
Fayetteville, North Carolina is a military town. Because of that, there are quite a lot of restaurants to choose from. Since I’m a BBQ fan, I got Patty to take me to a BBQ place while we were visiting the in-laws: Cape Fear BBQ & Chicken.
![[Cape Fear BBQ & Chicken]](http://whenchemistsattack.com/blogfiles/cfbbq_sign.jpg)
Cape Fear BBQ & Chicken sign
As you can see, this place is more or less a local joint. If you go to Cape Fear BBQ & Chicken, you will go for pork BBQ, not to admire the decor.
![[Cape Fear BBQ & Chicken]](http://whenchemistsattack.com/blogfiles/cfbbq_inside.jpg)
The view inside Cape Fear BBQ & Chicken
Prices are reasonable. A small BBQ plate – which comes with hush puppies and two sides – is $5.29. With drinks, our bill was less than $15.
![[Cape Fear BBQ & Chicken]](http://whenchemistsattack.com/blogfiles/cfbbq_2plates.jpg)
BBQ plates. These are the small BBQ plates. I hadn’t had any breakfast and one of these plates was more than enough for me.
The usual North Carolina sides are available: baked beans, Brunswick stew, slaw, potato salad, etc. The BBQ style is light vinegar – very much like the Village Inn in Lumberton.
Here’s a shot where you can see the BBQ.
![[Cape Fear BBQ & Chicken]](http://whenchemistsattack.com/blogfiles/cfbbq_plate.jpg)
BBQ plate close-up
So how was the BBQ? I have to again admit that vinegar BBQ is not my preferred taste, but Cape Fear BBQ serves up a respectable BBQ plate. I should also mention the hush puppies – not
If you’re passing through Fayetteville and are looking for some BBQ, Cape Fear BBQ & Chicken is worth a stop. You’ll find them at 523 Grove Street.
Looking for the perfect Christmas gift? Here’s something I found while shopping this evening.
![[Seabed World Lamp Lighting Move]](http://whenchemistsattack.com/blogfiles/seabedworld_1.jpg)
It’s the Seabed World Lamp Lighting Move!
If you’re a bit confused as to what this gift is, you’re … not alone. Thankfully, the side of the box will at least inform us of what we should not do with the Seabed World Lamp Lighting Move:
![[Seabed World Lamp Lighting Move warnings]](http://whenchemistsattack.com/blogfiles/seabedworld_2.jpg)
Warnings
Oh, darn! I was looking for something to go in the dusty place. Guess I’ll have to get something else!
Humans like the Christmas season for various reasons. For adults, there’s reuinions with family and friends. For kids, it’s toys, festive lights, snow, and a break from school.
For cats, it’s a tree. The tree is a place to play, where humans have attached the most wonderful cat toys.
And underneath the tree? A wonderful place to lay.
![[Merry Catmas!]](http://whenchemistsattack.com/blogfiles/tom_merrycatmas.jpg)
Tom relaxes under the tree
Merry Catmas from the shrimpandgrits family cats: Tom, Ash, and Rusty!
Scientific American has a brief article about industry’s request to have lead removed from the EPA’s list of regulated pollutants.
The EPA said that from 1980 to 2005 the national annual lead concentrations have dropped more than 90 percent. Lead levels in air have mostly fallen because it was banned as a gasoline additive starting in the 1970s.
This is good news, since exposure to lots of lead is no good thing.
But I am puzzled. How will removing lead from the list of regulated pollutants lead to less lead in the environment? (Sorry, couldn’t resist the pun!)
Who wants lead off the list?
In a letter last July to the EPA, industry group the Battery Council International urged the agency to “delete lead from the criteria pollutants.”
Who’s this “Battery Council International”?
Battery Council International is a not for profit organization with the mission to promote the interests of the international lead-acid battery industry.
Surely, the lead-acid battery industry will not pollute the environment with lead if we remove the regulations! Right?
They probably just want to turn it to gold. 🙂
I’ve often said to students that I’d rather be taking an exam than grading one. They sometimes stare back at me with jaws hanging open, shocked that I would say such a thing. In this post, I thought I’d take a brief look at a few of the reasons why grading an exam is harder than taking one.
Here’s a question from one of my recent exams for an introductory chemistry course.
Metallic aluminum (Al, FW = 26.98 g/mol) reacts with oxygen gas (O2, FW = 32.00 g/mol) to produce aluminum oxide (Al2O3, FW = 101.96 g/mol). How many grams of oxygen gas are required to react with 14.7 grams of aluminum metal?
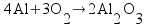
Based on the way I taught my students to solve this sort of problem in class (the factor-label method), I expected most students to come up with the answer 13.1 g of O2 using a calculation procedure similar to this one:
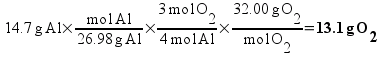
But there’s a hitch! There’s more than one way to work this problem. Another way to solve the problem is to find out the mass ratios of aluminum to oxygen based on the chemical equation and set it up as a ratio:
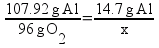
Solving, x = 13.1 g of O2.
This looks very different than the first way I showed to solve the problem. Though it’s not the way I taught chemical calculations, it’s a perfectly legitimate strategy for solving this kind of problem. To make it more … interesting, you can actually set up the ratios so that they look a little different from the way I wrote them above.
So, when you prepare to grade a test, you not only have to solve the problem the way you would have solved it yourself, but you also have to consider the problem-solving strategies your students might come up with to solve the problem. Otherwise, you won’t be able to see whether a student actually has an understanding of the material, and you won’t be able to help them correct any mistakes they made if you aren’t able to follow their strategies.
Think that’s bad? There’s another hitch!
Here is an actual student answer to the problem above.
8.72 g of O2 is needed to react with 1.47 g Al
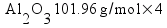
107.92 Al
96 O
53.96x = 440.4
8.7214.7g Al = ___________ g O2
12.45
(Yes, the answer really did look like that on paper.)
Obviously, the student got the wrong answer. Equally obviously, this “solution” is a mess! But, is there anything we can work with here in the mess? Is there any help we can give the student so that (s)he might do better next time?
It’s very clear that the student wanted to try using the ratio method to solve the problem. Why? There are actually two attempts to use the ratio method in this answer. The attempt on the right would have actually gotten the student to the right answer, but the student makes a math error in the attempt and comes up with 12.45 g of O2. Because the student doesn’t label what their numbers or letters represent, (s)he simply doesn’t realize that the value of x is the desired answer – and gets lost.
Once students start floundering in a sea of unfriendly numbers, they never end up anywhere useful. The wrong-on-all-counts ratio on the left-hand side of the page makes that point quite clearly. If the student had reazlied that the value of x in the ratio on the right was the desired answer, (s)he would have stopped at 12.45 g O2. Still not correct, but only off because of a case of fumble-fingers with the calculator.
The advice this student needs to hear, I think, is that when solving a problem it is vitally important to keep track of what numbers and variables actually represent. In math class, students find x for x‘s sake. Everywhere else, x is merely a name for sometihng real. This is a point that I don’t think is made nearly strongly enough in math classes.
But back to the title of this post – how long did it take to sift through the student answer? Longer, i’d wager, than the student spent on the exam solving the problem. And that’s why grading an exam is harder than taking one!
Here’s an update from the front lines of the War on Christmas. Rock Hill (SC) mother has 12-year-old son arrested for opening Christmas present early
The boy’s great-grandmother had told him not to open his Nintendo Game Boy Advance, which she had wrapped and placed beneath the Christmas tree, according to a police report.
Don’t open your present early, or Santa will throw your sorry behind in jail!
The boy was arrested on petty larceny charges, taken to the Rock Hill police station in handcuffs and held until his mother picked him up after church.
… because the reason for the season is using the cops as a babysitter while you go to church? Silly me. I thought it was something about giving.
Reading the article, I see that there’s a lot wrong with the situation: an uneducated single mother who’s 27 years old with two children ages 12 and 7, one of the kids being diagnosed with ADHD and facing expulsion from school, etc. But that’s no justification for trying to get your 12-year-old a police record to “scare” him – for trying to mess with his Christmas present early. That is a family issue, not a police issue.
(Hint: If it’s that important to keep the presents away from your kids, don’t put them under the tree until Christmas Eve.)
His mother said neither arrest seemed to scare him as she had hoped. She is distressed because her son is relishing the attention brought by his latest arrest.
Color me unsurprised.
Oh, and thie story seems to have hit CNN, too. I hate it when this is how South Carolina gets into the national press…