Cola drinks, as almost everyone knows, contain dissolved gas. The gas is present in two forms in the drink: dissolved CO2 molecules, and carbonic acid (H2CO3) – which forms in a reversible reaction between the carbon dioxide and water.
Dissolved CO2 is partially responsible for the flavor of colas, and is completely responsible for the fizz. The fizz is what we’re interested in for this blog post. Like all gases, carbon dioxide takes up a lot of space relative to its mass. When dissolved in liquid, it takes up less space than it would in the gas form. What if all the dissolved carbon dioxide in a bottle of cola were to come out of the liquid at once? The gaseous carbon dioxide would push against the liquid and the sides of the bottle. if there was a path for the liquid and gas to escape, they would shoot out rather rapidly.
You can get some dissolved carbon dioxide to come out of a cola by shaking it (who hasn’t seen this at least once?). This is good for practical jokes, but doesn’t make for an impressive fountain. For that, we need something better: Mentos mints.
While I’m not sure of the mechanism (I have a few ideas), Mentos mints catalyze the release of carbon dioxide from colas. Catalysts speed up a process, and Mentos mints make the carbon dioxide come out of cola fast. Really fast. Fast enough to blow three quarters of the liquid out of a two liter cola bottle.
We tried putting some Mentos into a two liter bottle of Diet Coke in my introductory chemistry class – it’s a good demonstration of the effects of gas pressure. Here are the results.
Click each image to enlarge.
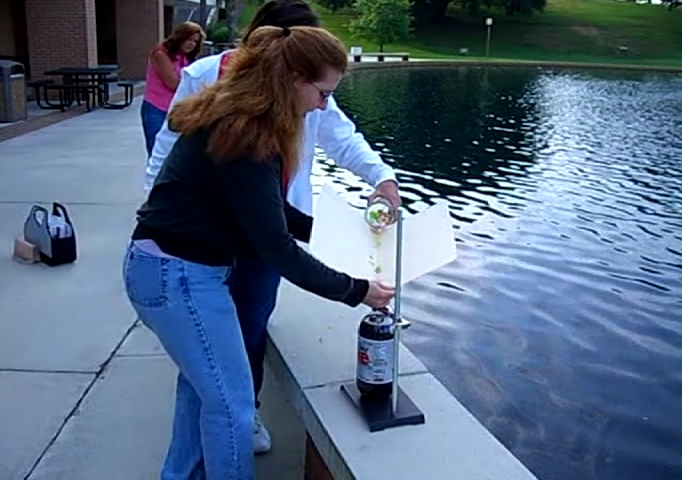
Loading the Mentos. I had the students use the folder as a chute to get the Mentos into the bottle because that way it would be less likely for the students to put their head directly over the bottle. (See? I’m not completely evil!)
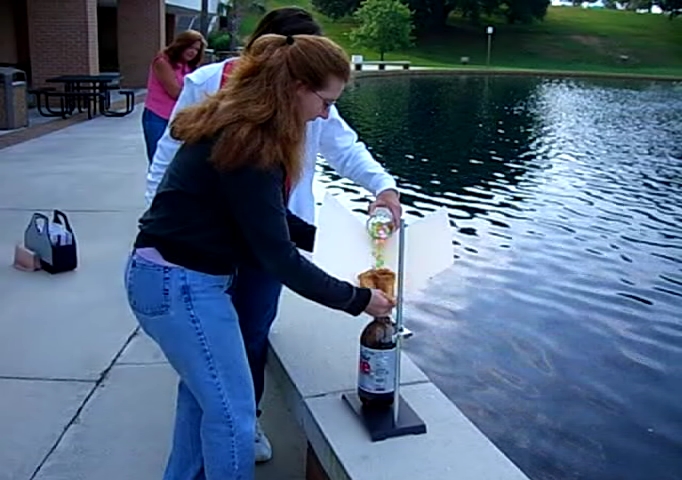
You can see the cola already starting to shoot out of the bottle. My students haven’t yet noticed, since this is only a few seconds after the first few Mentos make it into the bottle.
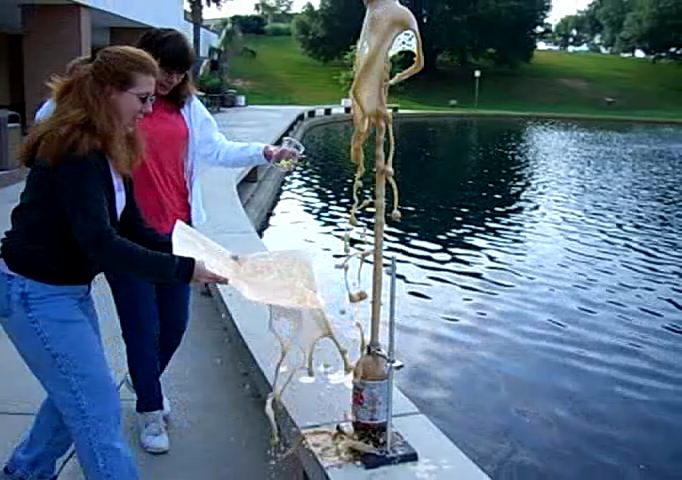
Behold! The mighty Coke fountain!
We estimate that cola shot up about four feet over the top of the bottle. This is similar to other results from around the web.

Is that the face of Jesus in the cola? Or is it something more sinister? (This experiment was performed on 06/06/06, after all!
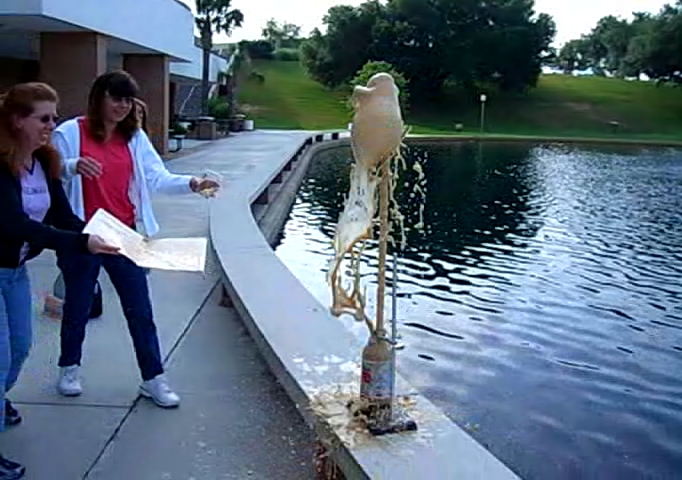
Who’s going to clean this up, anyway?
I think I’ll try this again with my other classes. It’s cheap, safe, entertaining, and requires no special hardware. Just don’t do it inside!

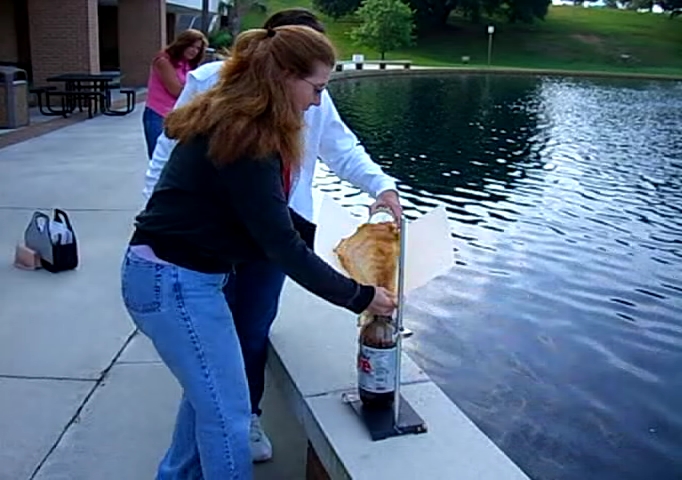
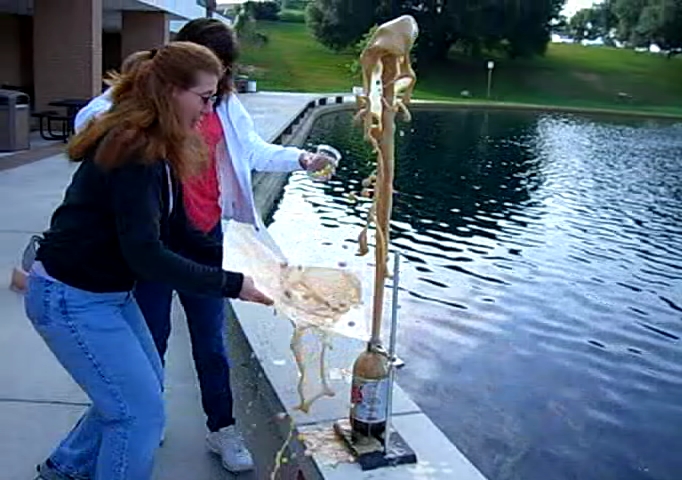
I agree with the entertaining part.
Hey, is that security code impossible to read, or am I just getting old? (trying again….)
I’ve noticed that the security code script occasionally spits out poor combinations of colors. This can usuallly be fixed by simply hitting “reload” on the browser, which will generate a new code with (hopefully) a better set of colors. I’d rather not have the thing at all, but the spambots were getting ridiculous – even with spam filters on.
I’m going to try to tweak the script to see if I can make it stop giving out hard-to-read color combinations, but I’m no expert in PHP scripting.
And yes, you probably are getting old. 🙂
6/14/06: Edited to add: I think I fixed the security script to stop giving out completely unreadable color combinations.
How many Mintos to a bottle?
I gave the students a whole roll, but it appears that only about five or six Mentos mints actually made it into the bottle.
Makes me think of urban legend of eating a combination of POp-Rocks and cola could make ones stomach explode. I really enjoy your site….
http://en.wikipedia.org/wiki/Pop_Rocks
All I saw was a big boom because we put the cap on it
How did you get the cap back on the bottle?
Ha, that is so coool!! I wish we had awesome experiments like this when I was in school. How do you make the fountain go higher though?
Don Lapre Fan
http://www.broklynresponds.com
bob@brooklynresponds.com
yeah i took a poop that exploded like that
I tried it in my garden
🙂 did you see coke in lot of acid
maybe i will put my web site.this is cool
🙂 no coke.t will drink pepsi