One of the many hats I wear here at the college is that of student adviser. On the surface, it seems a simple task; look at the student’s courses and make sure they stay on track for their major. But it’s less easy than it looks.
A colleague of mine was advising a student recently. I overheard, since all of the science department offices are clustered right next to one another. This student had declared nursing as a major, and had just finished our remedial English and math courses – making him ready to move on to introductory algebra and freshman composition and some freshman-level college courses.
To qualify for admission into the nursing program***, however, this student still needs to take a four semester sequence of introductory anatomy, college anatomy, physiology, and microbiology. There’s a small wrinkle; he’s attempted the introductory anatomy course one time already and had to drop because he wasn’t doing well. One more poor grade would drop him into academic probation.
So, this student is looking at no fewer that eight semesters until he can graduate with an associate’s degree in nursing and become an R.N. It’s going to be a long road for this student. For many in similar situations, it is an impossible road. Most students who drop out of our simplest anatomy course for academic reasons do not make it into the nursing program, and fewer still actually make it through.
Still, though, everyone deserves their shot. This is one of the students that you put into his courses, warn him that his performance in these introductory courses will determine if he gets into nursing or not, and suggest that there are other programs – like surgical technology**** – that he could get into and land a hospital job in year or so. And if he’s still gung-ho on nursing, you point out places – like the tutoring center – that will increase his chances of making it through his upcoming science classes.
So, my colleague starts figuring out what courses this student needed, and the student drops his bombshell. He wasn’t really interested in being a nurse. He was going to go to medical school and be a doctor. The nursing degree, he said, was just something to do until he became a doctor.
My colleague, sounding a bit surprised by the student’s revelation, suggests that the student might want to change his major to one of our degrees meant for college transfer. Most of the courses required to get an associate’s in nursing are specific to the program – and not accepted for credit at a university. At this point that the student becomes defensive and accuses my colleague of “being negative”. Apparently, the student doesn’t want to hear that the path they’ve chosen might not be the best way to reach his goal.
So what can you do with a student like this? Not a lot, I’m afraid. It makes me sad. This student sees us as an adversary – an obstacle they must jump over to reach his goal. That view will hurt him every time he steps into a classroom, and may very well contribute to his goals remaining forever out of reach.
***Our school has an open-door policy. Essentially, anyone who wants to come out here can come. Individual programs do have entry requirements, though. Even so there are usually multiple ways to get into a program. For nursing, students who don’t score high enough on the SAT can earn their way into the program by completing their required biology and math courses. After they have proven themselves, they’ll be accepted to the program.
****Surgical technology doesn’t pay as well as nursing, but it requires only a single anatomy course. Students who can’t handle the anatomy and microbiology requirements for nursing can often succesd in this program.

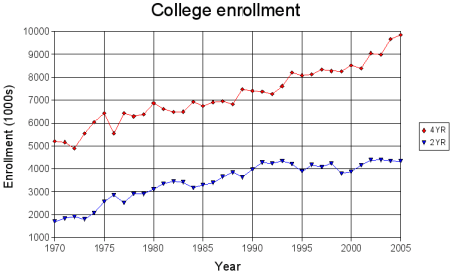
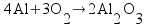
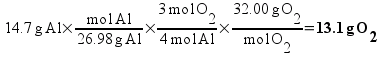
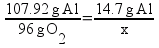
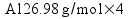
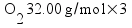
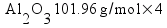
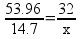
![[Dangerous things often look like nothing special - from Star Ocean: The Second Story for Playstation]](http://whenchemistsattack.com/blogfiles/so2_dangerousthings_tiny.jpg)