Here’s a comparison I did of the results of using graded homework assignments in a freshman-level chemistry class. The classes compared all had homework assignments given each week, but only some of them had assignments that were taken up, graded, and counted directly towards the final course grade.
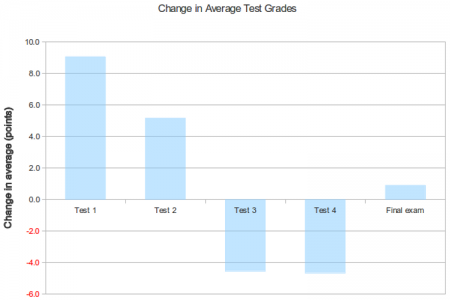
As you can see, there was a nearly a letter grade improvement in scores on the first test, compared to classes that didn’t have the graded assignments. There was an improvement on the second test as well, though it’s much more modest than the improvement on the first test. So the graded assignments appear to help students start off the course on a better footing than they would have otherwise.
However, the scores for the third and fourth tests are nearly half a letter grade lower than for the classes without the graded assignments. What’s the cause of this drop in performance? A few possibilities:
- Attrition may explain some of the falloff. Students who started off more poorly in the classes without the graded assignments are more likely to withdraw from the course earlier – leaving a greater percentage of higher-performing students behind for the last two test. (The latest a student is allowed to withdraw from a course is shortly after the third test.)
- Student participation in the graded assignments dropped somewhat as the semester progressed. More students skipped turning in homework before the third and fourth tests compared to the first and second.
- Students are more likely later in the semester to copy their assignments from other students of from tutors in the school’s tutoring center – depriving them of any benefit they might have gotten from doing the assignment.
For the final exam, the students in the graded assignments classes showed a small improvement over the others.
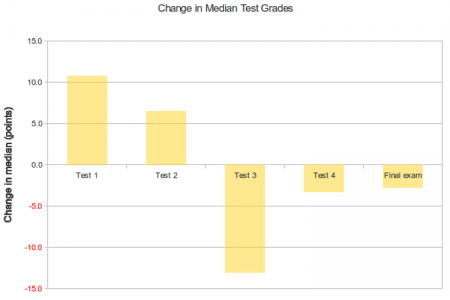
Since these classes are small sections as opposed to large auditorium classes, here’s a look at the median change in test scores for the same set of students.
The median scores follow roughly the same trends as the averages do – with better performance on the first two tests and poorer performance on the later tests. Interestingly, the median final exam scores for the students with graded assignments was slightly lower than the median final exam score for students without the graded assignments.


![[Regression line AND equation: OpenOffice 2.4.0]](http://whenchemistsattack.com/wp-content/uploads/2008/03/simplestats.jpg)
![[A = kc]](http://whenchemistsattack.com/wp-content/uploads/2008/03/akc.jpg)
![[The wrong plot]](http://whenchemistsattack.com/wp-content/uploads/2008/03/beerslawplot_html_5f969792.jpg)
![[The right plot]](http://whenchemistsattack.com/wp-content/uploads/2008/03/beerslawplot_html_510ea8d2.jpg)