A little while ago, about 384 hours if you want to be exact, I was in the lab doing an experiment with some of my students. The experiment involved isolating proteins from milk, then doing some chemical tests to show that what the students had isolated was actually protein.
One of the tests the students used on their isolated protein was the xanthoproteic test. This is a simple test for proteins that works on proteins containing an aromatic ring, and involves the addition of a nitro group to the ring by reaction with nitric acid. If a protein or amino acid with an aromatic ring is present, the test will give a yellow color.
You may, if you’ve ever been in an introductory chemistry lab, heard your teacher warn you about nitric acid. In addition to causing you some pain, it will also turn your skin or fingernails yellow. This is the same sort of chemistry in the xanthoproteic test. Your skin and nails, after all, contain proteins.
Now, back to 384 hours ago. While I was cleaning up the lab after my students had left, I spilled a small amount of nitric acid onto the top of my finger. Sure enough, my finger turned yellow, along with a small part of the fingernail.
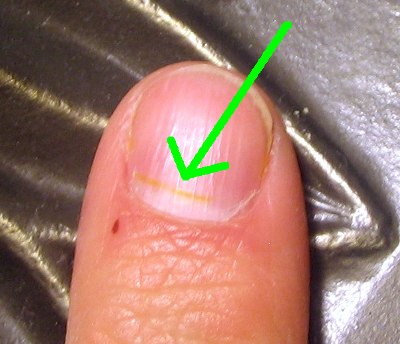
Here's the nitric acid stain. I originally spilled the acid at the point where my skin meets the fingernail. It's moved a bit, now.
384 hours after the spill, all the yellow skin had been replaced. But the nail has to grow out. We can find out how fast my fingernails are growing with a simple measurement.
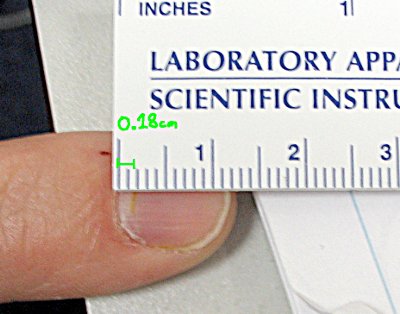
Here's a (somewhat crude) neasurment using a ruler from the lab. The nitric acid stain has moved about 0.18 cm.
So, my fingernail has been growing at a rate of (0.18 cm) / (384 hr) = 0.00047 cm per hour. That works out to be 3.4 mm per month, which is right about what Wikipedia claims for the average rate of fingernail growth.
Now who says you never learned anything useful from chemistry?

![[Liquid bromine and vapor] Liquid bromine and its vapor](http://whenchemistsattack.com/wp-content/uploads/2008/08/albr_before.jpg)
![[Aluminum bromide reaction, 10 seconds] Aluminum bromide reaction, 10 seconds after adding aluminum](http://whenchemistsattack.com/wp-content/uploads/2008/08/albr_still.jpg)
![[aluminum bromide sparks] After a few more seconds, it's hot enough to spark](http://whenchemistsattack.com/wp-content/uploads/2008/08/albr_still_2-450x316.jpg)
![[Aluminum bromide, flame] Oh yeah!](http://whenchemistsattack.com/wp-content/uploads/2008/08/albr_still_3-450x316.jpg)
![[Aluminum bromide: aluminum stuck to the beaker] Aluminum melted into the glass at the bottom of the beaker](http://whenchemistsattack.com/wp-content/uploads/2008/08/albr_aluminum.jpg)
![[Aluminum bromide solid on a beaker] Aluminum bromide (white / yellowish solid) on the beaker](http://whenchemistsattack.com/wp-content/uploads/2008/08/albr_product.jpg)