Homeopathy is an old form of “alternative” medicine that just doesn’t want to fade back into obscurity. One of the admittedly strange central ideas behind homeopathy is the idea that the more dilute a substance, the more potent it is. One homeopathy web site ( here ) explains it this way.
Key to the philosophy is the serial dilution of the remedies that get STRONGER “Biologically” as they get more dilute, or WEAKER from a “chemical” standpoint.
The problem with this line of thinking should be pretty obvious to anyone with some training in either biology or biochemistry – biology is, at the small scale, chemistry.
On the other hand, since many of the actve ingredients in homeopathic remedies are poisonous, perhaps making them weaker is no bad thing!
Homeopathic remedies are made by a process called serial dilution – which actually is a perfectly legitimate thing to do in a chemistry lab, as any student of analytical chemistry would be able to tell you. A serial dilution is just what it sounds like – repeated dilutions. Make a solution, then take a small portion of it and dilute it with water. Take a small portion of the new solution and dilute it with more water, and so on. The homeopaths add an extra step between each dilution, a kind of ritual shaking which they call succussion. The website referenced above tell us that this extra step
somehow energizes the remedy and adds “Necessary Energy” to the solution. Experiments have been done with hard physical measurements that have proven that the chemical bonds between the molecules actually get stronger.
Since they don’t say what kind of measurements have been made and where these results have been published, I’m not even going to attempt to evaulate this claim except to say that it’s unlikely based on what I know about the nature of chemical bonds. But even if there’s a small grain of truth in their claims, the homeopaths have another problem: there is a finite number of molecules in a solution. If you dilute a solution too much, you will eventually reach a point where a given volume of the solution is not likely to contain any molecules of the substance you are trying to dilute. Figuring this out should be simple enough for any student with a semester of freshman chemistry under their belt, and it’s an assignment I have given my freshman chemistry classes.
When I first made the assignment, I chose sulfuric acid as the substance to be diluted, mainly because it is a relatively simple molecule whose physical properties (like density) are easy to find. After doing a little more research, I find that sulfuric acid actually is used as a homeopathic remedy – going by the more impressive sounding name Sulphuricum Acidum. The homeopaths don’t seem to be in 100% agreement over the usage of sulfuric acid solutions, but they use it for treating weakness, trembling, skin discoloration, yellow stool, perspiration (after eating warm food), etc. (See here). Homeopathic sulfuric acid soltuions are available in a wide variety of dilutions, and it’s at this point that we need to define the system that homeopaths use to describe “potency” (in homeopathic language, that’s how dilute a solution is).
The system basically tells how many dilution steps were used, then follows it with a Roman numeral indicating the factor the solution was diluted by. For example, a 1X dilution contains one part (I’ll assume a volume here) of substance in ten parts of solution. The “1” indicates that only one dilution step was performed, while the “X” indicates that it’s a 1 in 10 solution. A 1L dilution contains 1 part of substance in 50 parts of solution. A 1C dilution contains 1 part of substance in 100 parts of solution … and so on.
If the number in front of the Roman numeral is bigger than 1, a serial dilution was performed. A 2X solution, for example, would take two steps to make: First prepare the 1X solution, then take one part of that solution and make another solution containing one part of “1X” solution per ten parts. With an “X” solution, each successive dilution decreases the concentration by a factor of ten. For a “C” solution, each successive dilution decreases the concentration by a factor of 100 … and so on.
Here’s where freshman chemistry comes in. Using calculations that any freshman chemistry student should be able to perform, it’s possible to calculate the approximate number of molecules contained in each solution after each dilution. Here’s how it’s done.
First, we assume that the first dilution is made from pure sulfuric acid in water. This may or may not be the starting point for the homeopathic preparations (they don’t say), but this would givw the largest number of molecules of sulfuric acid that the solutions could possibly have. If the homeopaths start from a solution of sulfuric acid instead of the pure subtance, their preparations will contain fewer molecules that we will calculate here.
Next, we’ll need the density of pure sulfuric acid, about 1.85 grams per milliliter at room temperature. We need this to figure out the mass (and later, the number of molecules) of sulfuric acid in the first solution. From a supplier of homeopathic remedies ( here ), we find that sulfuric acid is available in many concentrations. A few of them are 6C, 12C, 24C, and 30C. Our goal is to find out how much sulfuric acid is actually present in these solutions. The largest bottle available from this supplier is 50 mL, so we will do our calculation of the number of molecules based on this volume.
(If you’re not interested in the math, feel free to skip down a bit to the table.)
The simplest way to figure out the number of molecules in a bottle is to first change the “C” notation into a unit that actually relates simply to the number of molecules. In preparing the first dilution (1C), we would dissolve 1 mL of pure sulfuric acid into enough water to make 100 mL of solution. How much sulfuric acid, in terms of molecules, is that?
Since individual molecules are so small, chemical calculations are based on moles of molecules. A mole is simply a large number of molecules, 6.02 times 1023 ( 6.02×1023) of them. (This is no different than egg suppliers selling eggs by the dozen, or fireworks manufacturers selling firecrackers by the gross.) We will calculate the number of moles of sulfuric acid molecules in the 1C solution.
It is known that 98.09 grams of sulfuric acid contains one mole of sulfuric acid molecules (this is the called the molecular weight of sulfuric acid). We also know the density of the sulfuric acid, so we can calculate the number of moles of sulfuric acid in a milliliter.
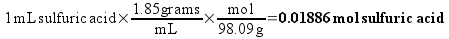
So, the 1C solution will contain 0.01886 moles of sulfuric acid in every 100 mL. Chemists routinely describe the concentration of solution in terms of molarity (abbreviated as M) – which is simply the number of moles of substance dissolved in a liter of solution. We have prepared 100 mL (0.100 L) of 1C solution, so the concentration is
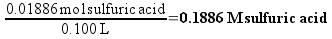
If the concentration of the 1C solution is 0.1886 M, then each liter of the solution would contain 1.13 times 1023 molecules (which sounds like a really large number, right? We’ll compare it to plain vinegar later). 50 mL (0.050 L) of the solution – the biggest bottle of homeopathic sulfuric acid available from our supplier – would contain 5.68 times 1021 molecules. This still seems like a really large number, but remember that molecules are really small.
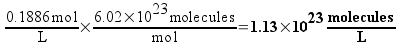
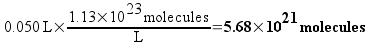
Each serial dilution requires one mL of the previous solution to be diluted to 100 mL. That means that each dilution will contain 1/100th of the number of molecules of sulfuric acid as the solution before it. Here’s a table of some dilutions of sulfuric acid and the number of molecules that a 50 mL bottle of each would (on average) contain.
| Dilution | Number of molecules in 50 mL |
| 1C | 5.68 times 1021 |
| 2C | 5.68 times 1019 |
| 6C | 5.68 times 1011 |
| 11C | 56.8 |
| 12C | 0.568 |
| 24C | 5.68 times 10-23 |
| 30C | 5.68 times 10-37 |
To put this in perspective, even the most concentrated (1C) dilution of sulfuric acid contains only about half the acid that the same amount of plain vinegar does (and that’s including the fact that sulfuric acid itself has two acidic protons while the acetic acid in vinegar only has one). So the 6C dilution is about 1/20,000,000,000th the strength of plain vinegar.
Another way to look at the numbers is to think about how many bottles of remedy you would have to buy to get a single molecule of sulfuric acid, For the 6C dilution, that’s not a problem – but for the 12C, 24C, and 30C solutions it is! If each bottle of 12C contains on average half a molecule of sulfuric acid, then you’d need to buy two bottles of remedy to be reasonably sure of getting a single molecule of active ingredient. That might not be too expensive, but you’d need about 2 times 1024 bottles of the 24C, and 2 times 1036 bottles of 30C. (Better open your wallet!)
Some homeopaths are at least a little honest about the fact that most of these bottles they sell contain nothing but water and perhaps some alcohol. Here’s what one web site ( here ) has to say on the matter.
Higher potencies of homeopathic remedies (anything higher than 12C) have been diluted past the point that molecules of the original substance would be measurable in the solution.
It’s not that the substanes aren’t detectable, it’s that quite likely a bottle will simply contain no molecules of the substance. Getting a molecule of the remedy, for the higher dilutions, would be like winning the Powerball. Occasionally, somebody wins – but how many people buy Powerball tickets and get nothing?
This is a major stumbling block for skeptics when it comes to understanding and accepting the idea of homeopathy.
Skeptics being defned as the set people who can do solution calculations?
Homeopathic remedies, when correctly chosen, clearly work—but not in the way that drugs do (through chemical actions that affect the body processes).
Or is it more likely that the most “potent” of the homeopathic medicines do not do anything at all and any “healing” that patients see is a result of the body’s ability to heal itself? Maybe some of this healing is simply the power of persuasion – people are told by a respected authority that thry’ll feel better, so they do.
If I bruise my arm, for instance, I could (if I weren’t very bright) decide that drinking one drop of toilet bowl cleaner dissolved in an eight ounce glass of water each day would make the bruise heal. If I did this, the toilet bowl cleaner probably wouldn’t do me any serious harm, and the bruise would heal in a matter of days. Can I claim that my “homeopathic toiletum bowlium cleanerum remedy” helped my arm to heal?
It is not completely understood why potentized remedies can work so deeply and specifically, but many likely theories have arisen through research and observation. It appears that they function on an energetic level to stimulate the body to heal itself more efficiently.
It is by no means proven that these remedies work at all, but look at the mechanism that is proposed: these remedies, many of which contain nothing at all, “function on an energetic level to stimulate the body”.
We have a name for things like that: the placebo effect.

This is a beautiful demonstration that I had to link to because of my frustration with homeopathy being lumped in with natural products and botanical medicines.
Just be careful – a homeopath might claim that you have created a remedy for burns caused by sulfuric acid.
Don’t know how I missed y’all previously but I’m a scientist who is a huge fan of shrimp and grits.
“somehow energizes the remedy and adds “Necessary Energy†to the solution.”
Yes, kinetic energy transferred to heat energy. Wow. Drink it while it’s hot.
“Experiments have been done with hard physical measurements that have proven that the chemical bonds between the molecules actually get stronger.”
Ignoring the obvious error, if the bonds got stronger, wouldn’t it make it harder for the body to break them down to actually use them? Sounds counterproductive to me.
Just found your site today…look forward to more!
In the case of homeopathic remedies like arsenic (“Arsenicum Album”), I wouldn’t want them interacting with my body in the first place. So perhaps this, too, is a good thing. 🙂
Coming up in a few weeks, my freshmen are doing an experiment that will show them that for acids, the fraction of acid that breaks down in water goes up as the concentration of the acid goes down. I’ll have to remind them about that homeopath claim about stronger bonds again then.